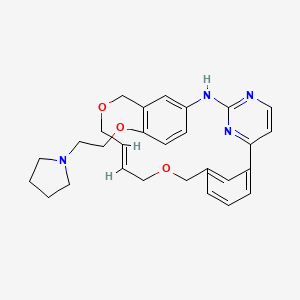
SB1578
ONX 0805
(9E)-15-(2-(Pyrrolidin-1-yl)ethoxy)-7,12,25-trioxa-19,21,24-triaza-tetracyclo[18.3.1.1(2,5).1(14,18)]hexacosa-1(24),2,4,9,14(26), 15,17,20,22-nonaene
7,12,26-Trioxa-19,21,24-triazatetracyclo[18.3.1.12,5.114,18]hexacosa-1(24),2,4,9,14,16,18(25),20,22-nonaene, 15-[2-(1-pyrrolidinyl)ethoxy]-, (9E)-
Phase 1 clinical trials
C26 H30 N4 O4
CAS 937273-04-6
CITRATE 1262279-15-1
HCL 1262279-16-2
S*Bio Pte Ltd INNOVATOR
SB1578 (disclosed in WO2007058627 and in WO2011008172 as the citrate salt) is in ongoing phase I studies for the treatment of rheumatoid arthritis. SB 1578 is shown below.

SB1578, also known as ONX-0805, is a novel, orally bioavailable JAK2 inhibitor with specificity for JAK2 within the JAK family and also potent activity against FLT3 and c-Fms. SB1578 blocks the activation of these kinases and their downstream signaling in pertinent cells, leading to inhibition of pathological cellular responses. The biochemical and cellular activities of SB1578 translate into its high efficacy in two rodent models of arthritis. SB1578 not only prevents the onset of arthritis but is also potent in treating established disease in collagen-induced arthritis mice with beneficial effects on histopathological parameters of bone resorption and cartilage damage. SB1578 abrogates the inflammatory response and prevents the infiltration of macrophages and neutrophils into affected joints. It also leads to inhibition of Ag-presenting dendritic cells and inhibits the autoimmune component of the disease. In summary, SB1578 has a unique kinase spectrum, and its pharmacological profile provides a strong rationale for the ongoing clinical development in autoimmune diseases. ( J Immunol. 2012 Oct 15;189(8):4123-34)
Synonym: ONX 0805; ONX0805; ONX0805; SB1578; SB1578; SB 1578.

PATENT
WO 2011008172
The compound 9E-15-(2-pyrrolidin-1-yl-ethoxy)-7,12,25-trioxa-19,21 ,24-triaza-tetracyclo[18.3.1.1(2,5).1(14,18)]hexacosa-1 (24),2,4,9,14,16l18(26)l20,22-nonaene (Compound I) was first described in PCT/SG2006/000352 and shows significant promise as a pharmaceutically active agent for the treatment of a number of medical conditions. Pharmaceutical development of this compound is underway based on the activity profiles demonstrated by the compound.

Compound I
In the development of a drug suitable for mass production and ultimately commercial use acceptable levels of drug activity against the target of interest is only one of the important variables that must be considered. For example, in the formulation of pharmaceutical compositions it is imperative that the pharmaceutically active substance be in a form that can be reliably reproduced in a commercial
manufacturing process and which is robust enough to withstand the conditions to which the pharmaceutically active substance is exposed.
From a manufacturing perspective, it is important that the commercial manufacturing process of a pharmaceutically active substance is such that the same material is produced when the same manufacturing conditions are used. In addition, it is desirable that the pharmaceutically active substance exists in a solid form where minor changes to the manufacturing conditions do not lead to major changes in the solid form of the pharmaceutically active substance produced. For example, it is important that the manufacturing process produces material having the same crystalline properties on a reliable basis, and also that the process produces material having the same level of hydration.
In addition, it is important that the pharmaceutically active substance be stable to degradation, hygroscopicity and subsequent changes to its solid form. This is important to facilitate the incorporation of the pharmaceutically active ingredient into pharmaceutical formulations. If the pharmaceutically active substance is hygroscopic (“sticky”) in the sense that it absorbs water over time it is almost impossible to reliably formulate the pharmaceutically active substance into a drug as the amount of substance to be added to provide the same dosage will vary greatly depending upon the degree of hydration. Furthermore, variations in hydration or solid form (“polymorphism”) can lead to changes in physico-chemical properties, such as solubility or dissolution rate, which can in turn lead to inconsistent oral absorption in a patient.
Accordingly, chemical stability, solid state stability, and “shelf life” of the pharmaceutically active agent are very important factors. In an ideal situation the pharmaceutically active agent and any compositions containing it, should be capable of being effectively stored over appreciable periods of time without exhibiting a significant change in the physico-chemical characteristics of the active component such as its activity, moisture content, solubility characteristics, solid form and the like.
In relation to 9E-15-(2-pyrrolidin-1-yl-ethoxy)-7,12,25-trioxa-19,21 ,24-triaza-tetracyclo[18.3.1.1 (2,5).1(14,18)]hexacosa-1(24),2,4,9,14,16,18(26),20,22-nonaene
initial studies were carried out on the hydrochloride salt and indicated that polymorphism was prevalent, with the compound being found to adopt more than one crystalline form depending upon the manufacturing conditions. In addition it was observed that the ratio of the polymorphs varied from batch to batch even when the manufacturing conditions remained constant. These batch-to-batch inconsistencies made the hydrochloride salt less desirable from a commercial viewpoint.
Accordingly it would be desirable to develop salts of 9E-15-(2-pyrrolidin-1-yl-ethoxy)-7, 12,25-trioxa-i 9,21 ,24-triaza-tetracyclo[18.3.1.1 (2,5).1 (14,18)]hexacosa-1(24)l2,4,9,14,16,18(26),20,22-nonaene which overcome or ameliorate one or more of the above identified problems.
Figure 22 shows a 1H NMR spectrum for Batch 4 in d6-DMSO.
Figure 23 shows a 1H NMR spectrum for Batch 4 in D2O.
List of hydrochloride and citrate salt batches used for comparative studies

Example 4 – Formation of the Citrate salt (Batch 4) in THF as solvent:
The free base of compound 1 (0.30Og, 0.648mmoles, 1.eq) was added to 12mL of THF. The solution was heated to reflux until complete dissolution was observed and maintained for 1h. A solution of citric acid (0.149g, 0.778mmoles, 1.2eq) dissolved in 12mL THF was then added slowly at reflux conditions. The mixture was refluxed for a further 15min then cooled. Crystallization was observed on gradual cooling. The crystals were stirred at room temperature for 12h and filtered under vacuum. The product was dried under vacuum to afford 250mg.
PATENT
Representative procedure for the synthesis of compounds type (XVIIIf)
5-(2-Chloro-pyrimidin-4-yl)-furan-2-carbaldehyde (XIIIfI) 

(XIIfI) (XIIIH) .
Compound (XIIIfI) was obtained using the same procedure described for compound (XIIIeI); LC-MS (ESI positive mode) /τVz 209 ([M+H]+)
[5-(2-Chloro-pyrimidin-4-yl)-furan-2-yl]-methanol (Xlllf2)

Compound (Xlllf2) was obtained using the same procedure described for compound (XXIb); LC-MS (ESI positive mode) m/z 211 ([M+H]+).
4-(5-Allyloxymethyl-furan-2-yl)-2-chloro-pyrimidine (XVfI)

Compound (XVfI) was obtained using the same procedure described for compound (XXIIb); LC-MS (ESI positive mode) m/z 251 ([M+H]+).
^-(S-Allyloxymethyl-furan-Σ-yO-pyrimidin^-yll-IS-allyloxymethyl^^-pyrrolidin-i-yl- ethoxy)-phenyl]-amine (XVIIfI)


(XVIb2) (XVIIfI)
Compound (XVIIfI) was obtained using the same procedure described for compound (XVIIbI); LC-MS (ESI positive mode) m/z 491.
Macrocycle Example 6 (Compound 38)

(XVIIfI)


Compound (38) was obtained using the same procedure described for compound (1) HPLC purity at 254nm: 99%; LC-MS (ESI positive mode) m/z 463 ([M+H]+); 1H NMR (MeOD-d4) δ 8.90 (d, 1 H), 8.33 (d, 1 H), 7.37 (d, 1 H), 7.17 (d, 1 H), 7.14-7.11 (m, 1 H)1 7.04 (d, 1 H), 6.67 (d, 1 H), 6.04 (dt, 1 H, CH, J = 5.2Hz, Jtrans = 15.8Hz), 5.96 (dt, 1 H, CH, J = 5.0Hz, Jtrans = 15.8Hz), 4.65 (s, 2H), 4.62 (s, 2H), 4.37 (t, 2H), 4.14 (d, 2H), 4.09 (d, 2H), 3.81 (br s, 2H), 3.66 (t, 2H), 3.33 (s, 2H), 2.21-1.98 (m, 4H).
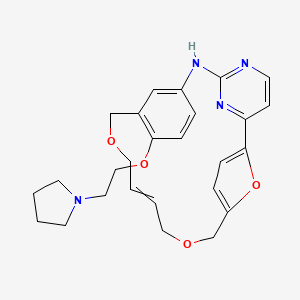
PAPER
Discovery of the Macrocycle (9E)-15-(2-(Pyrrolidin-1-yl)ethoxy)-7,12,25-trioxa-19,21,24-triaza-tetracyclo[18.3.1.1(2,5).1(14,18)]hexacosa-1(24),2,4,9,14(26),15,17,20,22-nonaene (SB1578), a Potent Inhibitor of Janus Kinase 2/Fms-LikeTyrosine Kinase-3 (JAK2/FLT3) for the Treatment of Rheumatoid Arthritis
S*BIO Pte. Ltd., 1 Science Park Road, #05-09 The Capricorn, Singapore Science Park II, Singapore 117528
J. Med. Chem., 2012, 55 (6), pp 2623–2640
DOI: 10.1021/jm201454n
Publication Date (Web): February 17, 2012
Copyright © 2012 American Chemical Society
*Tel: +65 62195443. E-mail: wanthony11@yahoo.com.

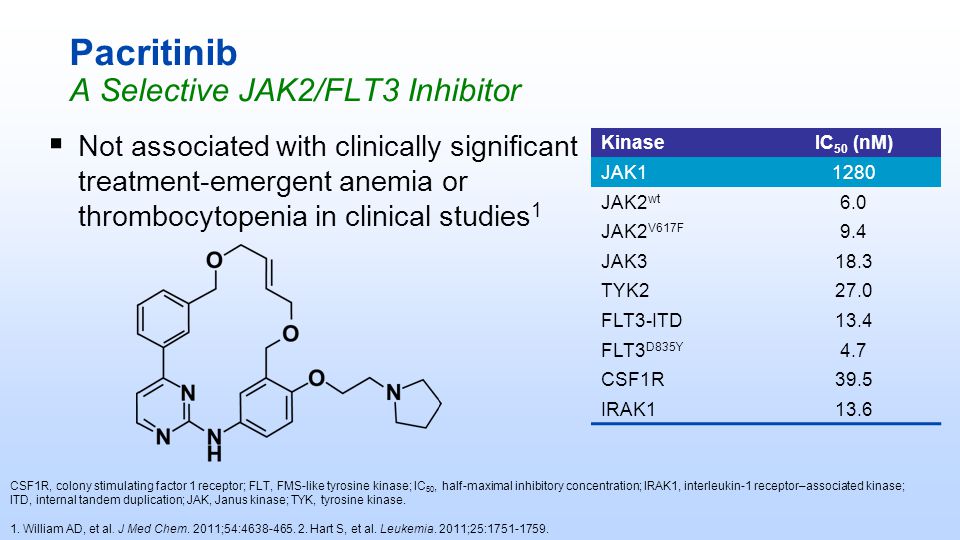
Herein, we describe the synthesis and SAR of a series of small molecule macrocycles that selectively inhibit JAK2 kinase within the JAK family and FLT3 kinase. Following a multiparameter optimization of a key aryl ring of the previously described SB1518 (pacritinib), the highly soluble 14l was selected as the optimal compound. Oral efficacy in the murine collagen-induced arthritis (CIA) model for rheumatoid arthritis (RA) supported 14l as a potential treatment for autoimmune diseases and inflammatory disorders such as psoriasis and RA. Compound 14l (SB1578) was progressed into development and is currently undergoing phase 1 clinical trials in healthy volunteers.
(9E)-15-(2-(Pyrrolidin-1-yl)ethoxy)-7,12,25-trioxa-19,21,24-triaza-tetracyclo[18.3.1.1(2,5).1(14,18)]hexacosa-1(24),2,4,9,14(26), 15,17,20,22-nonaene (14l)
The title compound was synthesized from 12n (yield, 46%; mixture of cis/trans 33:67 by 1H NMR).
LC-MS (ESI positive mode) m/z 474 ([M + H]+);
1H NMR (MeOD-d4) δ 8.91 (d, 1H), 8.57–8.54 (m, 1H), 8.28 (d, 1H), 7.70 (s, 1H), 7.51–7.46 (m, 1H), 7.38–7.32 (m, 1H), 7.14–7.12 (m, 1H), 7.05 (s, 1H), 5.93–5.85 (m, 1H), 5.68–5.62 (m, 1H), 4.46 (s, 2H), 4.58 (m, 2H), 4.46–4.34 (m, 2H), 4.12 (d, 2H), 3.82 (m, 2H), 3.72 (m, 2H), 3.37 (m, 2H), 2.52 (m, 2H), 2.25 (m, 2H), 2.10 (m, 2H).
REF
Madan B, Goh KC, Hart S, William AD, Jayaraman R, Ethirajulu K, Dymock BW, Wood JM. SB1578, a novel inhibitor of JAK2, FLT3, and c-Fms for the treatment of rheumatoid arthritis. J Immunol. 2012 Oct 15;189(8):4123-34. doi: 10.4049/jimmunol.1200675. Epub 2012 Sep 7. PubMed PMID: 22962687.
2: Poulsen A, William A, Blanchard S, Lee A, Nagaraj H, Wang H, Teo E, Tan E, Goh KC, Dymock B. Structure-based design of oxygen-linked macrocyclic kinase inhibitors: discovery of SB1518 and SB1578, potent inhibitors of Janus kinase 2 (JAK2) and Fms-like tyrosine kinase-3 (FLT3). J Comput Aided Mol Des. 2012 Apr;26(4):437-50. doi: 10.1007/s10822-012-9572-z. Epub 2012 Apr 22. PubMed PMID: 22527961.
3: William AD, Lee AC, Poulsen A, Goh KC, Madan B, Hart S, Tan E, Wang H, Nagaraj H, Chen D, Lee CP, Sun ET, Jayaraman R, Pasha MK, Ethirajulu K, Wood JM, Dymock BW. Discovery of the macrocycle (9E)-15-(2-(pyrrolidin-1-yl)ethoxy)-7,12,25-trioxa-19,21,24-triaza-tetracyclo[18. 3.1.1(2,5).1(14,18)]hexacosa-1(24),2,4,9,14(26),15,17,20,22-nonaene (SB1578), a potent inhibitor of janus kinase 2/fms-like tyrosine kinase-3 (JAK2/FLT3) for the treatment of rheumatoid arthritis. J Med Chem. 2012 Mar 22;55(6):2623-40. doi: 10.1021/jm201454n. Epub 2012 Mar 6. PubMed PMID: 22339472.
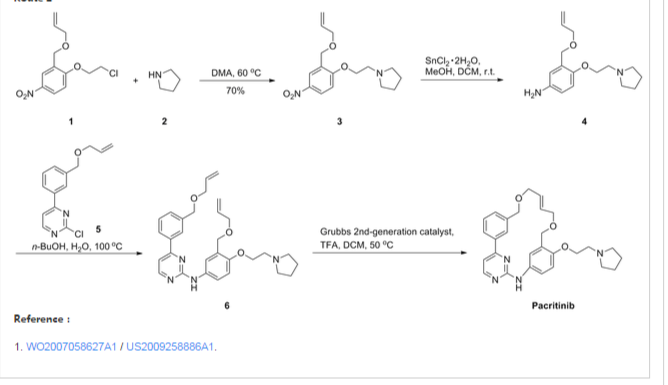
| WO2007058627A1 * | 15 Nov 2006 | 24 May 2007 | S Bio Pte Ltd | Oxygen linked pyrimidine derivatives |
| SG2006000352W | Title not available |


S*Bio Pte Ltd
Address: 1 Science Park Rd, Singapore 117528
Phone:+65 6827 5000
S*BIO Pte Ltd. provides research and clinical development services for small molecule drugs for the treatment of cancer in Singapore. The company’s products include JAK2 inhibitors, such as SB1518 for leukemia/myelofibrosis, lymphoma, and polycythemia; and SB1578 for RA/psoriasis. The company also offers SB939, a histone deacetylases for MDS/AML+combo, prostate cancer, sarcoma, pediatric tumor, and myelofibrosis; SB2602, a mTOR inhibitor; SB2343, a mTOR/PI3K inhibitor; and SB1317, a CDK/Flt3 inhibitor. The company was founded in 2000 and is based in Singapore. S*BIO Pte Ltd. operates as a subsidiary of Chiron Corporation Limited.
Highlights
• Principle lead and inventor of 3 clinical stage candidates,
1) SB1518 (Pacritinib)-A selective JAK2 inhibitor for myleofibrosis into phase 2,
2) SB1317 (TG02)-A mutikinase inhibitor CDK, JAK2, FLT3, and ERK5 into phase 1 and
3) SB1578-A more selective JAK2 inhibitor than pracritinib for autoimmune diseases such as Rheumatoid Arthritis (RA) and Psoriasis into phase 1
• Principle lead and inventor of 3 clinical stage candidates,
1) SB1518 (Pacritinib)-A selective JAK2 inhibitor for myleofibrosis into phase 2,
2) SB1317 (TG02)-A mutikinase inhibitor CDK, JAK2, FLT3, and ERK5 into phase 1 and
3) SB1578-A more selective JAK2 inhibitor than pracritinib for autoimmune diseases such as Rheumatoid Arthritis (RA) and Psoriasis into phase 1
N3=C1NC(=CC=N1)c2oc(cc2)COCC=CCOCc5cc3ccc5OCCN4CCCC4
OR
C1(C2=CC=C(O2)COC/C=C/COCC3=CC(N4)=CC=C3OCCN5CCCC5)=NC4=NC=C1