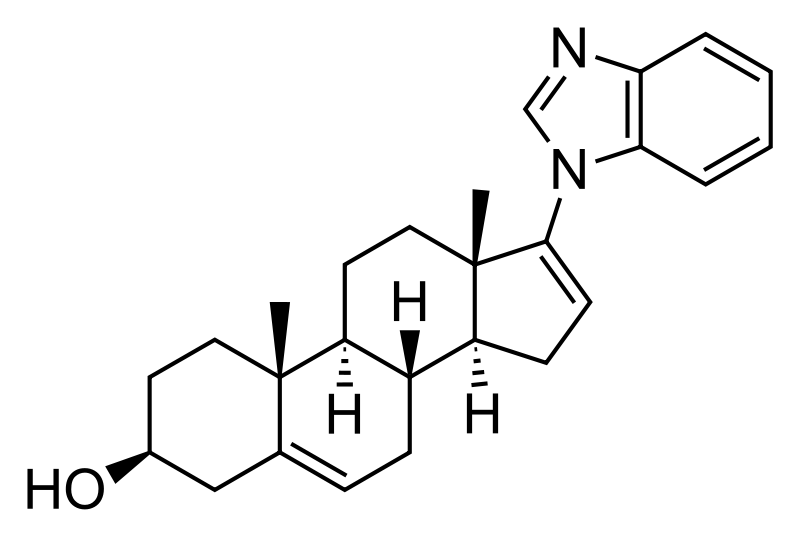
Galeterone
SYNTHESIS SEE BELOW
A SARM potentially for the treatment of prostate cancer.
Research Code, TOK-001; VN; 124; 124-1; 1241
TOK-001; Galeterone; 851983-85-2; VN/124; UNII-WA33E149SW; VN/124-1;
CAS No. 851983-85-2(Galeterone)
(3S,8R,9S,10R,13S,14S)-17-(benzimidazol-1-yl)-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15-decahydro-1H-cyclopenta[a]phenanthren-3-ol
Fast track 2012 f
| Molecular Formula: | C26H32N2O |
|---|---|
| Molecular Weight: | 388.54508 g/mol |

Galeterone (TOK-001 or VN/124-1) is a novel steroidal antiandrogen under development by Tokai Pharmaceuticals for the treatment of prostate cancer. It possesses a unique dual mechanism of action, acting as both an androgen receptor antagonist and an inhibitor of CYP17A1, an enzyme required for the biosynthesis of the androgens.[1] It shows selectivity for 17,20-lyase over 17-hydroxylase.[2]
As of 2016, galeterone is being compared to enzalutamide in a phase III clinical trial (ARMOR3-SV) for AR-V7-expressing metastatic castration-resistant prostate cancer.[3][4]
Specific Androgen Receptor Modulator CYP17 Inhibitor TOK-001 is an orally bioavailable small-molecule androgen receptor modulator and CYP17 lyase inhibitor with potential antiandrogen activity. Galeterone exhibits three distinct mechanisms of action: 1) as an androgen receptor antagonist, 2) as a CYP17 lyase inhibitor and 3) by decreasing overall androgen receptor levels in prostate cancer tumors, all of which may result in a decrease in androgen-dependent growth signaling. Localized to the endoplasmic reticulum (ER), the cytochrome P450 enzyme CYP17 (P450C17 or CYP17A1) exhibits both 17alpha-hydroxylase and 17,20-lyase activities, and plays a key role in the steroidogenic pathway that produces progestins, mineralocorticoids, glucocorticoids, androgens, and estrogens.
About Galeterone
We are conducting ARMOR3-SV, a Phase 3 clinical trial of galeterone evaluating whether administration of galeterone results in a statistically significant increase in radiographic progression-free survival as compared to Xtandi® (enzalutamide), an oral therapy currently approved for the treatment of CRPC, in AR-V7 positive metastatic CRPC patients. ARMOR3-SV is the first pivotal trial in prostate cancer to employ a precision medicine approach for patient selection. For more information regarding ARMOR3-SV, click here.
Galeterone has been studied in over 250 subjects in Phase 1 and Phase 2 clinical trials, including in CRPC patients with and without the AR-V7 splice variant. In these trials, galeterone demonstrated good tolerability and showed clinically meaningful reductions in levels of prostate specific antigen, or PSA, a biochemincal marker used to evaluate prostate cancer patients for signs of response to therapy.
We are currently focusing our late-stage development of galeterone on AR-V7 positive metastatic CRPC patients because it represents an unmet need in prostate cancer and our precision medicine approach provides an efficient development path. Based on the data we and our collaborators have produced to date, we also believe there is rationale for the broader clinical exploration of galeterone in the future.
Galeterone acts by disrupting the androgen receptor signaling pathway. This pathway is activated by the binding of male hormones (also known as androgens), such as testosterone and dihydrotestosterone (DHT) to androgen receptors in prostate cancer cells.
Galeterone disrupts the activation of the androgen receptor pathway in three ways:
- Androgen receptor degradation, which reduces the amount of androgen receptor protein in tumor cells. There are no currently marketed drugs whose mechanism of action entails degradation of the androgen receptor. Therefore, galeterone represents a potential first-in-class therapeutic opportunity.
- CYP17 enzyme inhibition, which blocks the synthesis of testosterone. This mechanism has been validated clinically by Zytiga (abiraterone). Zytiga must be co-administered with the steroid prednisone in order to minimize the risk of a potentially fatal side effect called mineralocorticoid excess. Unlike Zytiga, galeterone has not been shown in clinical trials to cause mineralocorticoid excess and, as a result, does not require co-administration of steroids. As a result, we believe that galeterone may be easier to administer, provide convenience for patients and enhance patient compliance.
- Androgen receptor inhibition, which blocks the binding of testosterone or DHT with the androgen receptor. This mechanism has been validated clinically by Xtandi® (enzalutamide), which is also currently approved for the treatment of CRPC. Xtandi™ has shown a risk of grand mal seizures in clinical trials. We have not had any reports of seizures in clinical trials of galeterone and, therefore, galeterone may have certain safety advantages over Xtandi.
Tokai retains global rights to galeterone. We intend to commercialize galeterone in the United States on our own, and to seek a partner to further develop and commercialize galeterone outside of the United States.
Galeterone has been granted Fast Track designation by U.S. Food and Drug Administration for the treatment of CRPC. Fast Track designation is designed to facilitate the development and expedite review of drugs intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
Figure 3: The structures of abiraterone, orteronel and galeterone.
From CYP17 inhibitors—abiraterone, C17,20-lyase inhibitors and multi-targeting agents
- Nature Reviews Urology 11,32–42 (2014)
- doi:10.1038/nrurol.2013.274

SYNTHESIS
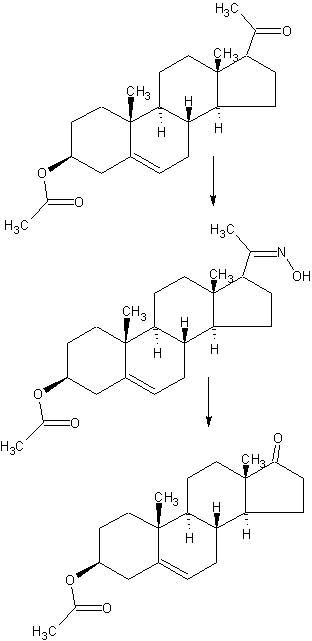
CN 104098638
DETAILED DESCRIPTION
1J loss reaction.
(1) raw material specifications to match.
acetate pregnancy dehydropregnenolone: toluene + ethanol: Batch steep: hydrochloric acid amine light = 1: 3: 0 4: 0.213, which pregnenolone acetate pregnancy 160kg, toluene + ethanol 320kg + 160kg, approved Steep 64kg, hydrochloric acid amine light 34kg.
(2) process operation.
In the first input 1000L tank oximation with hydroxylamine hydrochloride in pyridine, and then pumped into a mixed solvent of toluene and ethanol, the reaction solution was stirred and heated to complete dissolution, pregnancy-dehydropregnenolone acetate was added and heated under reflux for 3 hours, cooling and crystallization, The Department conducted into the centrifuge centrifugal drying, apply a recovery from the mother liquor, rinse with warm water mixture to no foam, centrifugal drying, drying to a moisture at 0.2% or less, that acetic acid in pregnancy dehydropregnenolone oxime (oxime compounds) 163kg, content of 99%, a melting point of 202-204 ° C, a yield of about 102% (for pregnenolone acetate pregnancy weight ratio).
2, heavy drain hydrolysis reaction.
(1) raw material specifications to match.
acetate pregnancy dehydropregnenolone waning: Benzene: Batch steep: phosphorus oxychloride and toluene: HCl + water = 1: 6 5: 0 4: 1: 3.5, which acetate pregnancy alcohol one hand 163kg, benzene 1060kg, batch steep 64kg, phosphorus oxychloride and toluene 80kg + 80kg, hydrochloric acid + water 245kg + 325kg.
(2) process operation.
The first drying 2000L rearrangement reaction tank, then pumped to the reaction tank benzene, alcohol into acetate pregnancy oxime, pulls out into benzene, stirring heated to reflux until the reaction mixture is completely dissolved, cooling to 1 (TC When, pyridine, of the reaction liquid at temperatures down to 6 ° C, start dropping a mixed solution of previously prepared phosphorus oxychloride and toluene (1: 1 mass ratio), slowly dropping, dropping control, first After slow fast reaction when dropping liquid temperature control in 4-8 ° C, the addition was complete, the reaction solution at 9-12 ° C for 3 hours the first time under.
After incubation, a solution has been a mixed solution of hydrochloric acid and water, good preparation, while dropping the reaction liquid temperature is controlled at 15-25 ° C, the addition was complete, the reaction solution at 15-25 ° C under a second Insulation 1. 5-2 hours. After incubation, stand 40 minutes, then points to lower acidic water layer, the remaining upper layer was added 0.3 times the amount of 30-35 ° C in the brine and let stand 20 minutes, a second watershed, sub lower aqueous layer was then allowed to stand for 30 minutes, a third water diversion, to give the final weight of the upper layer reaction solution was drained.
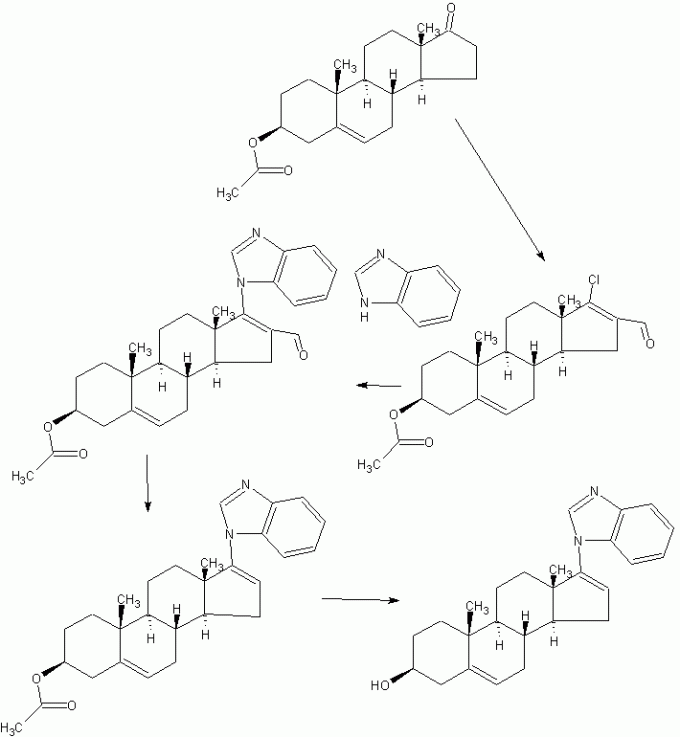
3, the red Dingding steam distillate process.
The rearrangement reaction liquid was pumped to punch distillate tank, conduct atmospheric distillate punch, has been rushed to the reaction mixture was distilled benzene mixed solvent only, at the start of the steam valve not to open too much, so as not to rush material, distillation after cooling discharge, centrifugal drying, washing with tap water to neutral, and then into the oven dried to a moisture in the square. 5% acetic acid in dehydroepiandrosterone (rearrangement thereof) The crude product is about 142kg, content of about 97.5%, a melting point of 160 ° C _165 ° C or so, yield about 88% (for acetate pregnancy dehydropregnenolone weight ratio).
4, refining processes.
The drying in acetic acid Dehydroepiandrosterone crude into refined tin, adding 8 times the weight of the crude methanol and 0.10 times the weight of activated carbon, heat, stirring to dissolve, reflux billion. 5 hours, filtered , concentrated, cooled to about 5 ° C, the discharge
1J loss reaction.
(1) raw material specifications to match.
acetate pregnancy dehydropregnenolone: toluene + ethanol: Batch steep: hydrochloric acid amine light = 1: 3: 0 4: 0.213, which pregnenolone acetate pregnancy 160kg, toluene + ethanol 320kg + 160kg, approved Steep 64kg, hydrochloric acid amine light 34kg.
(2) process operation.
In the first input 1000L tank oximation with hydroxylamine hydrochloride in pyridine, and then pumped into a mixed solvent of toluene and ethanol, the reaction solution was stirred and heated to complete dissolution, pregnancy-dehydropregnenolone acetate was added and heated under reflux for 3 hours, cooling and crystallization, The Department conducted into the centrifuge centrifugal drying, apply a recovery from the mother liquor, rinse with warm water mixture to no foam, centrifugal drying, drying to a moisture at 0.2% or less, that acetic acid in pregnancy dehydropregnenolone oxime (oxime compounds) 163kg, content of 99%, a melting point of 202-204 ° C, a yield of about 102% (for pregnenolone acetate pregnancy weight ratio).
2, heavy drain hydrolysis reaction.
(1) raw material specifications to match.
acetate pregnancy dehydropregnenolone waning: Benzene: Batch steep: phosphorus oxychloride and toluene: HCl + water = 1: 6 5: 0 4: 1: 3.5, which acetate pregnancy alcohol one hand 163kg, benzene 1060kg, batch steep 64kg, phosphorus oxychloride and toluene 80kg + 80kg, hydrochloric acid + water 245kg + 325kg.
(2) process operation.
The first drying 2000L rearrangement reaction tank, then pumped to the reaction tank benzene, alcohol into acetate pregnancy oxime, pulls out into benzene, stirring heated to reflux until the reaction mixture is completely dissolved, cooling to 1 (TC When, pyridine, of the reaction liquid at temperatures down to 6 ° C, start dropping a mixed solution of previously prepared phosphorus oxychloride and toluene (1: 1 mass ratio), slowly dropping, dropping control, first After slow fast reaction when dropping liquid temperature control in 4-8 ° C, the addition was complete, the reaction solution at 9-12 ° C for 3 hours the first time under.
After incubation, a solution has been a mixed solution of hydrochloric acid and water, good preparation, while dropping the reaction liquid temperature is controlled at 15-25 ° C, the addition was complete, the reaction solution at 15-25 ° C under a second Insulation 1. 5-2 hours. After incubation, stand 40 minutes, then points to lower acidic water layer, the remaining upper layer was added 0.3 times the amount of 30-35 ° C in the brine and let stand 20 minutes, a second watershed, sub lower aqueous layer was then allowed to stand for 30 minutes, a third water diversion, to give the final weight of the upper layer reaction solution was drained.
3, the red Dingding steam distillate process.
The rearrangement reaction liquid was pumped to punch distillate tank, conduct atmospheric distillate punch, has been rushed to the reaction mixture was distilled benzene mixed solvent only, at the start of the steam valve not to open too much, so as not to rush material, distillation after cooling discharge, centrifugal drying, washing with tap water to neutral, and then into the oven dried to a moisture in the square. 5% acetic acid in dehydroepiandrosterone (rearrangement thereof) The crude product is about 142kg, content of about 97.5%, a melting point of 160 ° C _165 ° C or so, yield about 88% (for acetate pregnancy dehydropregnenolone weight ratio).
4, refining processes.
The drying in acetic acid Dehydroepiandrosterone crude into refined tin, adding 8 times the weight of the crude methanol and 0.10 times the weight of activated carbon, heat, stirring to dissolve, reflux billion. 5 hours, filtered , concentrated, cooled to about 5 ° C, the discharge
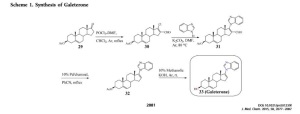
JMC 2005, 48, 2972-84
The most potent CYP17 inhibitors were 3β-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene (5, code named VN/124-1), 3β-hydroxy-17-(51-pyrimidyl)androsta-5,16-diene (15)
PAPER
JMC 1998 41, 902-12
The most potent compounds are 3β-hydroxy-17-(1H-imidazol-1-yl)androsta-5,16-diene (17), 3β-hydroxy-17-(1H-1,2,3-triazol-1-yl)androsta-5,16-diene (19), and 17-(1H-imidazol-1-yl)androsta-4,16-dien-3-one (28), with Ki values of 1.2, 1.4, and 1.9 nM, respectively,
Discovery and Development of Galeterone (TOK-001 or VN/124-1)
for the Treatment of All Stages of Prostate Cancer.......http://pubs.acs.org/doi/pdf/10.1021/jm501239f
CLICK ON PIC FOR CLEAR VIEW

| Patent ID | Date | Patent Title |
|---|---|---|
| US2011034428 | 2011-02-10 | Treatment of Prostate Cancer |
| US7875599 | 2011-01-25 | C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens, in vitro biological activities, pharmacokinetics and antitumor activity |
| US2010137269 | 2010-06-03 | Novel C-17-Heteroaryl Steroidal Cyp17 Inhibitors/Antiandrogens: Synehesis, In Vitro Biological Activities, Pharmacokinetics and Antitumor Activity |
| US2010048914 | 2010-02-25 | Novel C-17-Heteroaryl Steroidal Cyp17 Inhibitors/Antiandrogens, In Vitro Biological Activities, Pharmacokinetics and Antitumor Activity |
| US2010048913 | 2010-02-25 | Novel C-17-Heteroaryl Steroidal CYP17 Inhibitors/Antiandrogens Synthesis In Vitro Biological Activities, Pharmacokinetics and Antitumor Activity |
| US2010048912 | 2010-02-25 | Novel C-17-Heteroaryl Steroidal CYP17 Inhibitors/Antiandrogens, In Vitro Biological Activities, Pharmacokinetics and Antitumor Activity |
| US2010048524 | 2010-02-25 | Novel C-17-Heteroaryl Steroidal CYP17 Inhibitors/Antiandrogens Synthesis In Vitro Biological Activities, Pharmacokinetics and Antitumor Activity |
| US2010047338 | 2010-02-25 | Novel C-17-Heteroaryl Steroidal CYP17 Inhibitors/Antiandrogens, In Vitro Biological Activities, Pharmacokinetics and Antitumor Activity |
| Patent ID | Date | Patent Title |
|---|---|---|
| US2013336962 | 2013-12-19 | AZIRIDINE BISPHENOL ETHERS AND RELATED COMPOUNDS AND METHODS FOR THEIR USE |
| US8569393 | 2013-10-29 | UV-LED curable compositions and inks |
| US2013203615 | 2013-08-08 | ANTIANDROGEN THERAPY MONITORING METHODS AND COMPOSITIONS |
| US2012309861 | 2012-12-06 | PHOTOINITIATORS FOR UV-LED CURABLE COMPOSITIONS AND INKS |
| US2012237502 | 2012-09-20 | METHOD FOR TREATING BREAST CANCER AND OVARIAN CANCER |
| US2011319369 | 2011-12-29 | COMBINATION OF A 17 ALPHA-HYDROXYLASE/C17, 20-LYASE INHIBITOR WITH AN ADDITIONAL THERAPEUTIC AGENT |
| US2011312924 | 2011-12-22 | NOVEL STEROIDAL CYP17 INHIBITORS/ANTIANDROGENS |
| US2011312916 | 2011-12-22 | NOVEL PRODRUGS OF STEROIDAL CYP17 INHIBITORS/ANTIANDROGENS |
| US2011118219 | 2011-05-19 | NOVEL PRODRUGS OF C-17-HETEROARYL STEROIDAL CYP17 INHIBITORS/ANTIANDROGENS: SYNTHESIS, IN VITRO BIOLOGICAL ACTIVITIES, PHARMACOKINETICS AND ANTITUMOR ACTIVITY |
| US2011105445 | 2011-05-05 | ANDROGEN RECEPTOR INACTIVATION CONTRIBUTES TO ANTITUMOR EFFICACY OF CYP17 INHIBITORS IN PROSTATE CANCER |
| Patent ID | Date | Patent Title |
|---|---|---|
| US2015051179 | 2015-02-19 | NOVEL STEROIDAL CYP17 INHIBITORS/ANTIANDROGENS |
| US2015005265 | 2015-01-01 | METHODS AND COMPOSITIONS FOR COMBINATION THERAPY USING P13K/MTOR INHIBITORES |
| US2014371261 | 2014-12-18 | INDOMETHACIN ANALOGS FOR THE TREATMENT OF CASTRATE-RESISTANT PROSTATE CANCER |
| US2014371181 | 2014-12-18 | NOVEL PRODRUGS OF STEROIDAL CYP17 INHIBITORS/ANTIANDROGENS |
| US2014343024 | 2014-11-20 | TREATMENT OF PROSTATE CANCER |
| US2014288037 | 2014-09-25 | NOVEL COMPOSITIONS AND METHODS FOR TREATING PROSTATE CANCER |
| US2014288036 | 2014-09-25 | NOVEL C-17-HETEROARYL STEROIDAL CYP17 INHIBITORS/ANTIANDROGENS, IN VITRO BIOLOGICAL ACTIVITIES, PHARMACOKINETICS AND ANTITUMOR ACTIVITY |
| US2014274983 | 2014-09-18 | NOVEL PRODRUGS OF C-17-HETEROARYL STEROIDAL CYP17 INHIBITORS/ANTIANDROGENS: SYNTHESIS, IN VITRO BIOLOGICAL ACTIVITIES, PHARMACOKINETICS AND ANTITUMOR ACTIVITY |
| US2014107085 | 2014-04-17 | Bifunctional AKR1C3 Inhibitors/Androgen Receptor Modulators and Methods of Use Thereof |
| US2013336962 | 2013-12-19 | AZIRIDINE BISPHENOL ETHERS AND RELATED COMPOUNDS AND METHODS FOR THEIR USE |
| CN101691392A * | Sep 17, 2009 | Apr 7, 2010 | 扬州市天平化工厂有限公司 | Method for preparing 3beta-acetoxyl group-5androstene-17ketone |
| CN102212099A * | Apr 2, 2011 | Oct 12, 2011 | 邵阳市科瑞化学品有限公司 | Synthesis method for dehydroepiandrosterone |
| CN102603839A * | Jan 13, 2012 | Jul 25, 2012 | 宜城市共同药业有限公司 | Preparation method of dehydroepiandrosterone |
| CN102746356A * | Jul 17, 2012 | Oct 24, 2012 | 湖北芳通药业股份有限公司 | Process for producing dehydroepiandrosterone acetate through homogeneous phase method |
| Reference | ||||
|---|---|---|---|---|
| 1 | * | 石诚等: "5-雄甾烯-3β-醇-17-酮-3-醋酸酯的工艺研究", 《山东化工》, vol. 41, no. 1, 31 December 2012 (2012-12-31) | ||
| 2 | * | 石诚等: "醋酸妊娠双烯醇酮肟的工艺研究", 《广州化工》, vol. 39, no. 23, 31 December 2011 (2011-12-31), pages 78 - 79 | ||
References
- 1 Brawer MK (2008). "New treatments for castration-resistant prostate cancer: highlights from the 44th annual meeting of the american society of clinical oncology, may 30-june 3, 2008, chicago, IL". Rev Urol 10 (4): 294–6. PMC 2615106. PMID 19145273.
- 2
- Yin L, Hu Q (2014). "CYP17 inhibitors--abiraterone, C17,20-lyase inhibitors and multi-targeting agents". Nat Rev Urol 11 (1): 32–42. doi:10.1038/nrurol.2013.274. PMID 24276076.
- 3
- "A Study of Galeterone Compared to Enzalutamide In Men Expressing Androgen Receptor Splice Variant-7 mRNA (AR-V7) Metastatic CRPC - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-02-27.
- 4
 | |
| Systematic (IUPAC) name | |
|---|---|
17-(1H-benzimidazol-1-yl)androsta-5,16-dien-3β-ol
| |
| Clinical data | |
| Routes of administration | Oral |
| Identifiers | |
| CAS Number | 851983-85-2 |
| PubChem | CID 11188409 |
| ChemSpider | 9363493 |
| KEGG | D10125 |
| Chemical data | |
| Formula | C26H32N2O |
| Molar mass | 388.25 |
C[C@]12CC[C@@H](CC1=CC[C@@H]3[C@@H]2CC[C@]4([C@H]3CC=C4N5C=NC6=CC=CC=C65)C)O
CC12CCC(CC1=CCC3C2CCC4(C3CC=C4N5C=NC6=CC=CC=C65)C)O
 Androgen receptor degradation, which reduces the amount of androgen receptor protein in the tumor cells.
Androgen receptor degradation, which reduces the amount of androgen receptor protein in the tumor cells. Androgen receptor antagonism, which blocks the binding of testosterone or DHT with the androgen receptor.
Androgen receptor antagonism, which blocks the binding of testosterone or DHT with the androgen receptor. Inhibition of the enzyme CYP17, which blocks the synthesis of testosterone.
Inhibition of the enzyme CYP17, which blocks the synthesis of testosterone.



Galeterone (TOK-001 or VN/124-1) is a novel antiandrogen under development by Tokai Pharmaceuticals for the treatment of prostate cancer. It possesses a unique dual mechanism of action, Galeterone
ReplyDelete